Our equity story
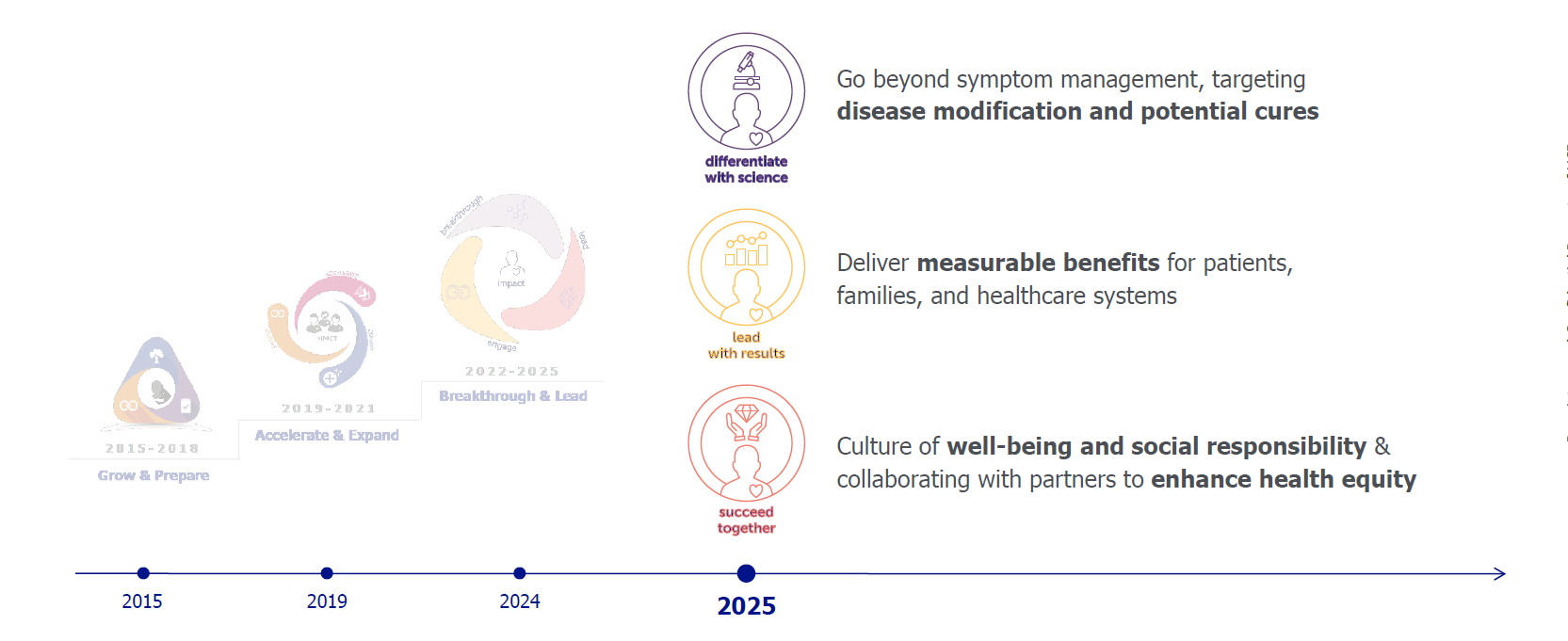
UCB’s Decade+ of Growth: Elevating lives of people through our medicines
Our key medicines
We bring solutions to people living with neurological or immunological diseases.
Growth drivers
BIMZELX® (bimekizumab)

Reaching more than 49 700 patients globally (Dec 2024)
Indications: Psoriasis (PSO); Psoriatic Arthritis (PsA); Ankylosing spondylitis (AS); non-radiographic Axial Spondyloarthritis (nr-axSpA)
Loss of Exclusivity (indicative): 2035 in U.S., without patent term extension; 2036 in Europe, 2037 in Japan
Sales: € 607 million in 2024
Peak sales guidance: > € 4 billion
EVENITY® (romosozumab)

Reached more than 900 000 patients globally since launch (Dec 2024)
Indication: Osteoporosis
Loss of Exclusivity (indicative): 2031 in Europe and Japan, 2033 in U.S.
Sales: € 103 million in 2024 in Europe. Net sales outside Europe reported by Amgen and Astellas
FINTEPLA® (fenfluramine)

Reaching more than 7 600 patients globally (Dec 2024)
Indications: Dravet Syndrome, Lennox-Gastaut Syndrome.
Loss of Exclusivity (indicative). 2032 in Europe and Japan, 2033 in U.S.
Sales: € 340 million in 2024.
Peak sales guidance: € 800 million by 2027.
RYSTIGGO® (rozanolixizumab)

Launched in the U.S. in July 2023, approved and launched in Europe and Japan
Indication: generalized Myasthenia Gravis.
Loss of Exclusivity (indicative): 2033 in Japan. 2034 in Europe and 2035 in U.S., all without patent term extension.
Sales: € 202 million in 2024.
ZILBRYSQ® (zilucoplan)
Global launches started April 2024
Indication: generalized Myasthenia Gravis.
Loss of Exclusivity (indicative): 2035 in Europe, Japan & U.S. without patent term extension.
Solid foundation
BRIVIACT® (brivaracetam)

Reaching more than 230 000 patients globally (MAT Q3/20234)
Indication: Epilepsy partial-onset seizure, also known as focal seizure
Loss of Exclusivity (indicative): 2026 in Europe & U.S.
Sales: € 686m in 2024, reaching its peak sales two years ahead of 2026
Peak sales guidance: ≥ € 600 million by 2026
CIMZIA® (certolizumab pegol)

Reaching more than 180 000 patients globally (MAT Q3/2023)
Indications: Ankylosing spondylitis (AS); non-radiographic Axial Spondyloarthritis (nr-axSpA); Crohn's disease (CD); Psoriasis (PSO); Psoriatic arthritis (PsA); Rheumatoid arthritis (RA)
Loss of Exclusivity (indicative): 2024 in Europe & U.S. 2026 in Japan.
Sales: € 2 033 million in 2024
Peak sales guidance: ≥ € 2 billion by 2024 achieved in 2022
KEPPRA® (levetiracetam)

Reaching more than 1.8 million patients globally (MAT Q3/2024)
Indications: Epilepsy partial-onset seizures, also known as focal seizures; Epilepsy primary generalized tonic-clonic seizures; Epilepsy myoclonic seizures
Loss of Exclusivity: U.S. - 2008, Europe - 2010, Japan - 2020.
Sales: € 582 million in 2024
Peak sales: € 1.2 billion (2008)
NAYZILAM® (midazolam nasal spray)

Reaching more than 93 000 patients in the U.S. (As of December 2024)
Indication: Epilepsy seizure clusters
Loss of Exclusivity (indicative): 2028 in U.S.
Sales: € 124 million in 2024
VIMPAT® (lacosamide)

Reaching more than 570 000 patients globally (MAT Q3/2024)
Indications: Epilepsy partial-onset seizures, also known as focal seizures; Epilepsy primary generalized tonic-clonic seizures
Loss of Exclusivity: 2022 in Europe & U.S. 2024 in Japan (indicative)
Sales: € 329 million in 2023
Peak sales: € 1.5 billion (2021)
Our clinical development partnerships
Amgen
Biogen
Novartis
Cancer Research UK
Our clinical development pipeline
UCB continuously innovates and strives to find new ways to deliver solutions to people living with severe immunological and neurological diseases, reflected in a clinical development pipeline encompassing now one phase 4 (post-approval) asset, one asset under regulatory review, four phase 3 projects, four phase 2 projects - addressing different patient populations.
-
bimekizumab (IL-17 A/F)
MODALITY: Monoclonal antibody
THERAPEUTIC AREA: Immunology
INDICATION: Post-approval head-to-head study versus risankizumab in PsA
PHASE:
INFO: Phase 4 - H2 2026
-
doxecitine and doxribtimine (nucleoside therapy)
MT1621 is an investigational therapy that combines two small molecules, deoxycytidine (dC) and deoxythymidine (dT). It targets the underlying pathophysiology of Thymidine kinase 2 deficiency (TK2d) by restoring mitochondrial DNA (mtDNA) replication fidelity.
MODALITY: Small molecule
THERAPEUTIC AREA: Neurology
INDICATION: Thymidine kinase 2 deficiency (TK2d)
PHASE: 3
INFO: Filed
-
rozanolixizumab (FcRn inhibitor)
Rozanolixizumab is an investigational humanized monoclonal antibody that specifically binds to human neonatal Fc receptor (FcRn). It has been designed to block the interaction of FcRn and IgG, inhibiting IgG recycling and inducing the removal of pathogenic IgG autoantibodies.
MODALITY: Monoclonal antibody
THERAPEUTIC AREA: Neurology
INDICATION: Myelin oligodendrocyte glycoprotein (MOG) antibody disease
PHASE: 3
INFO: H2 2026
-
fenfluramine (5-HT agonist)
Fenfluramine is an investigational serotonin releasing agent, that has shown to stimulate multiple 5-HT receptor sub-types through the release of serotonin. Fenfluramine may reduce seizures by acting as an agonist at specific serotonin receptors in the brain, including the 5-HT1D, 5-HT2A, and 5-HT2C receptors, and also by acting on the sigma-1 receptor
MODALITY: Small molecule
THERAPEUTIC AREA: Neurology
INDICATION: CDKL5 deficiency disorder
PHASE: 3
INFO: H1 2025
-
dapirolizumab pegol (anti-CD40L antibody)
Dapirolizumab pegol is an investigational humanised monovalent pegylated Fab antibody fragment against the CD40 ligand (CD40L). Through interactions with its receptor, CD40, CD40L plays an important role in regulating interactions between T cells and other immune cells and thus affects several important functional events thought to be involved in autoimmune disease.
Dapirolizumab pegol is being co-developed with Biogen. 1st phase 3 study.
MODALITY: Monoclonal antibody
THERAPEUTIC AREA: Immunology
INDICATION: Systemic lupus erythematosus
PHASE: 3
INFO: 1st positive Phase 3 - 2nd Phase 3: 2028
-
STACCATO® alprazolam (benzodiazepine)
STACCATO® alprazolam is an investigational drug-device combination using STACCATO® delivery technology with alprazolam, a benzodiazepine, that has the potential to be the first rescue treatment to be administered by a patient or caregiver in an out-patient setting to rapidly terminate (within 90 seconds) an ongoing seizure.
MODALITY: Small molecule
THERAPEUTIC AREA: Neurology
INDICATION: Stereotypical prolonged seizures
PHASE: 3
INFO: H1 2026
-
bepranemab (anti-tau antibody)
Bepranemab is an investigational recombinant, humanised, full length IgG4 monoclonal anti-tau antibody with specificity for human tau protein.
Bepranemab is being co-developed with Roche/Genentech
MODALITY: Monoclonal antibody
THERAPEUTIC AREA: Neurology
INDICATION: Alzheimer's disease
PHASE: 2
INFO: Positive Phase 2a
-
UCB0022 (D1 receptor positive allosteric modulators)
THERAPEUTIC AREA: Neurology
INDICATION: Parkinson's disease
PHASE: 2
INFO: H1 2025
-
UCB9741/galvokimig (IL-17 A/F & IL-13)
-
MODALITY: -
THERAPEUTIC AREA: Immunology
INDICATION: Atopic dermatitis
PHASE: 2
INFO:Positive Phase 2a
-
UCB1381 / donzakimig (IL-13 & IL-22)
-
MODALITY: -
THERAPEUTIC AREA: Immunology
INDICATION: Atopic dermatitis
PHASE: 2
INFO:H2 2025
